Quantum

science chemistry experiment states of matter Fundamental Photographs
#1 viper2308 19 0 Homework Statement Which of the following electron configurations correspond to an excited state? Identify the atoms and write the ground-state electron configuration where appropriate. 1s2 2s2 2p4 3s1 [Ar]4s2 3d5 4p1 Homework Equations none The Attempt at a Solution I have no idea what to do.

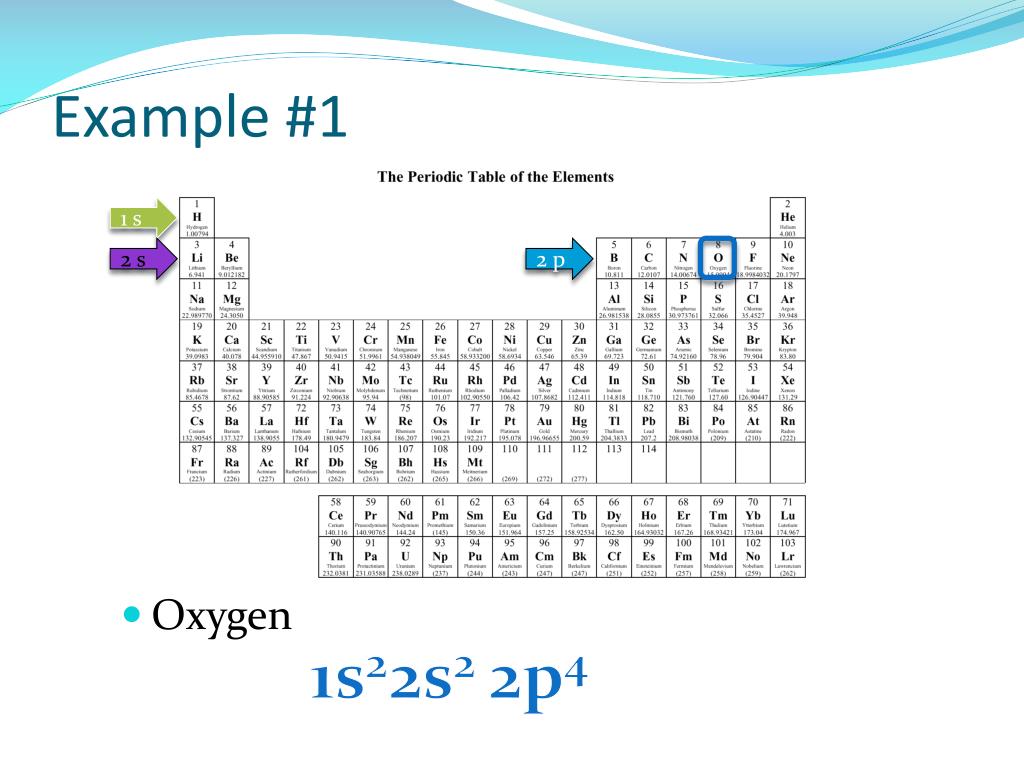
The electronic configuration for oxygen is written as 1s2 2s2 2p4
Oxygen's electron configuration is 1s2 2s2 2p4. We know it has 8 total electrons, and 6 in its outermost shell. Just as with nitrogen, the 1s and 2s orbitals will be filled, but we need to use Hund's Rule to fill the 2p orbital correctly.

science chemistry experiment states of matter Fundamental Photographs
We would like to show you a description here but the site won't allow us.

1s2 2s2 2p4 Which element has the electron configuration of 1s2 2s2
Sulfur is a nonmetal element with an atomic number of 16. This means that it has 16 protons in its nucleus. The sulfur electron configuration lists the different ways that sulfur can arrange its electrons. The most common sulfur electron configuration is 1s2 2s2 2p6 3s2 3p4. This means that the sulfur atom has two electrons in the first energy.

science chemistry experiment states of matter Fundamental Photographs
1s2 2s2 2p4 ||Which element has the electron configuration of 1s2 2s2 2p4 ?#1s22s22p4
Periodic table f serrealt
Which element has the electron configuration of 1s2 2s2 2p4 ? Wayne Breslyn 727K subscribers Join Subscribe Subscribed 157 23K views 3 years ago To figure this out the element with the electron.

2S1 2/2 YouTube
These guides are subject to and governed by the General Statutes of the State of North Carolina. N.C. Department of State Treasurer, Retirement Systems Division 3200 Atlantic Avenue, Raleigh, North Carolina 27604 1-877-NCSECURE (1-877-627-3287) toll-free www.myncretirement.com GUIDES.
PPT Atomic Structure and Bonding PowerPoint Presentation, free
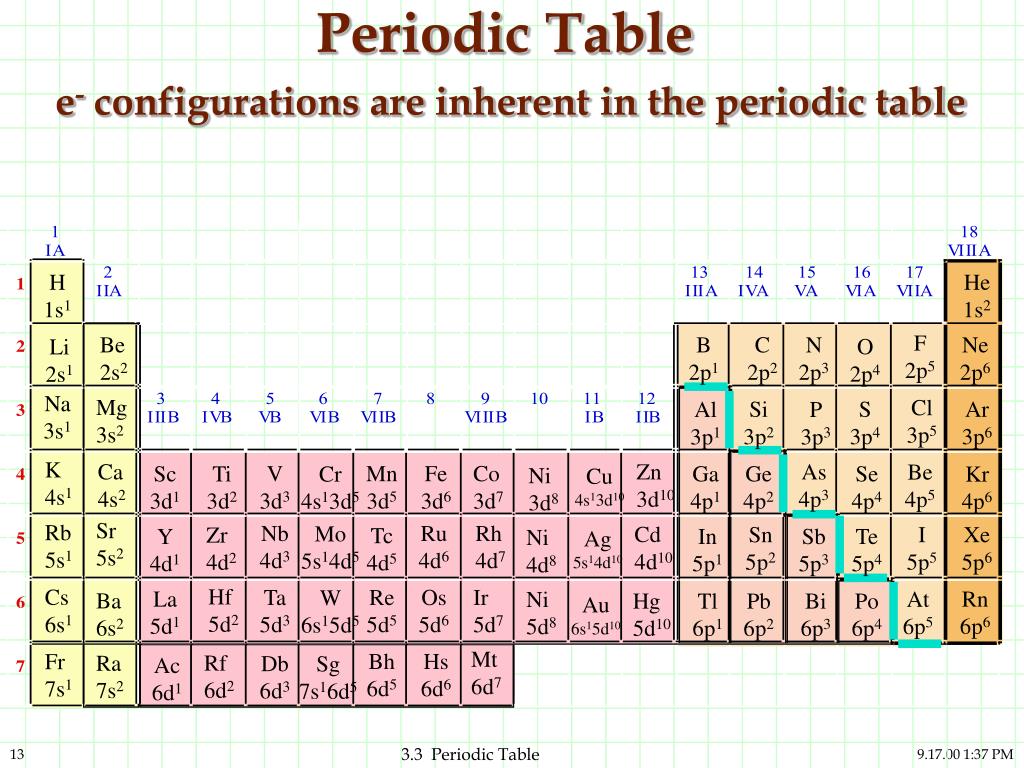
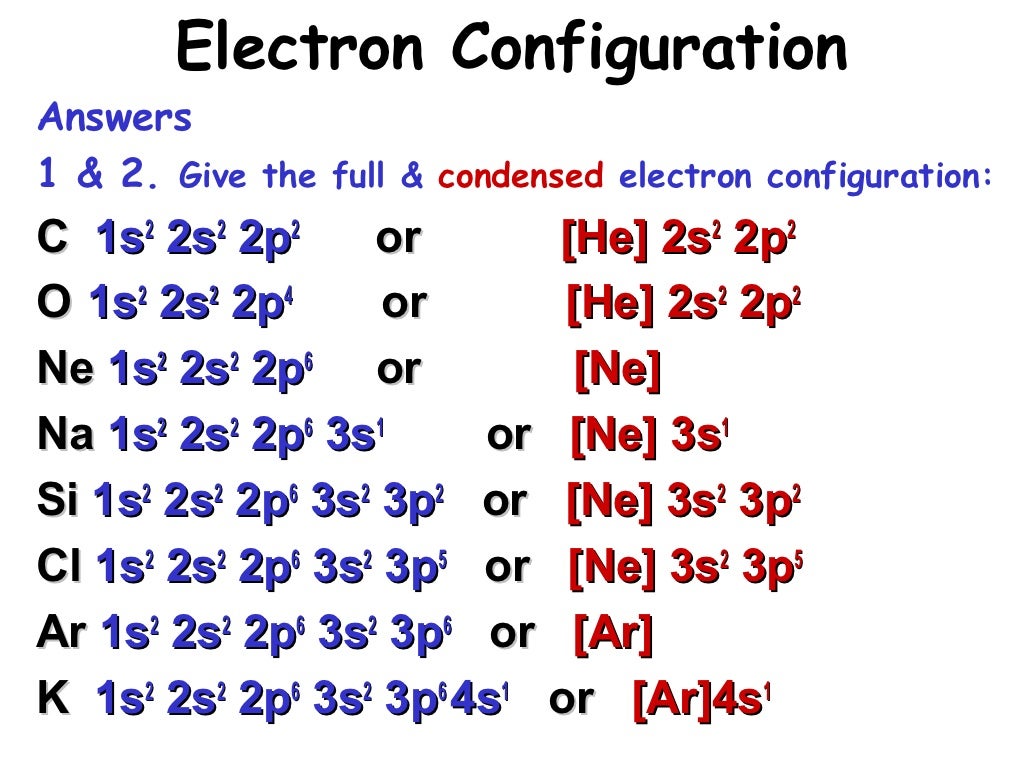
Question Electronic configuration of first 30 elements with table full explanation Solution H (Hydrogen) 1s1 He (Helium) 1s2 Li (Lithium) 1s2 2s1 Be (Beryllium) 1s2 2s2 B (Boron) 1s2 2s2 2p1 C (Carbon) 1s2 2s2 2p2 N (Nitrogen) 1s2 2s2 2p3 O (Oxygen) 1s2 2s2 2p4 F (Fluorine) 1s2 2s2 2p5 Ne (Neon) 1s2 2s2 2p6 Na (Sodium) 1s2 2s2 2p6 3s1

science chemistry experiment states of matter Fundamental Photographs
This Electron Configuration Full table gives the Electron Configuration Full of all the elements of periodic table . Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Electron Configuration Full ' headers to sort. Periodic Table of Elements with Electron Configuration Full Trends

Orbital Diagram for Oxygen JessicaknoeWilkinson
What is the atomic number of the element with an electron configuration of 1s2 2s2 2p4? ? 3 ? 4 ? 8 ? 13; What element has the configuration 1s2 2s2 2p1?. How many inner-shell electrons does the following configuration indicate: 1s2 2s2 2p6 3s2 3p3? ? 3 ? 10 ? 12 ? 15; Which letter is used to indicate the angular momentum quantum number?.

1s2 2s2 2p4 What element has the electron configuration of 1s2 2s2
The ground-state electron configurations of the elements are listed in Table 9.9.9 B. 1. The "exceptions" to the simple mnemonic noted in general chemistry texts are partly a consequence of the inadequacy of a "one-orbital order-fits-all" model. For example, copper has an electron configuration of [Ar]4s 1 d 10.
[Solved] Na O I I S Si 1s2 2s2 2p4 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2
For example, the electronic configuration of oxygen (atomic number 8) is 1s2 2s2 2p4. This indicates that oxygen has two electrons in the 1s subshell, two electrons in the 2s subshell, and four electrons in the 2p subshell.. indicating that it has 12 electrons. The electronic configuration of magnesium is: 1s2 2s2 2p6 3s2. This configuration.

Chemistry291 Hand Note 【 】 1s2 2s2 2p4 What element has the electron
What element has the electron configuration of 1s2 2s2 2p6 3s2 3p4? Sulfur (S). You can sum the number of electrons on all the subshells, and you will get the number 16. In its ground state, it corresponds to an atomic number that is unique to sulfur in the periodic table.
Quantum
About Meet Halfway. Meet Halfway is an independently produced service for approximating the halfway point along a mapped route. Made by Sean with the help of Google Maps APIs.Write me here.. Third Party Cookies. This website uses Google services like Google Analytics and Google Maps APIs, some which have their own use of cookies and cookie policies.

PPT Chapter 7 The Structure of Atoms and Periodic Trends PowerPoint
Sulfur is a 'group 16' element (electronic configuration: [Ne] 3s2 3p4) and Oxygen is also a 'group 16' element (electronic configuration: 1s2 2s2 2p4) in Periodic table. To draw the electron dot structure we count all the outer most shell electrons that participate in the molecule formation. Both Sulfur and Oxygen has two less.

science chemistry experiment states of matter Fundamental Photographs
Key Questions How do electron configurations correspond to the periodic table? When looking at electron configuration, your fill order of electrons is: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s etc. Group 1A (1), the alkali metals all end is s1. What period the element is in determines the 1st number.